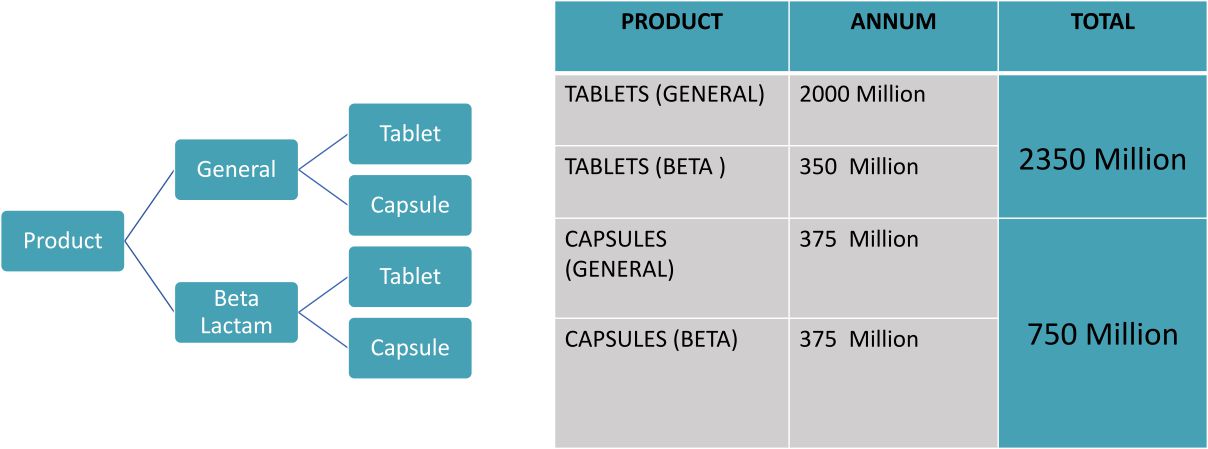
To serve our domestic and international markets, our manufacturing facilities , which has been created following the WHO GMP guidelines , has separate quality control units and quality assurance functions to observe the manufacturing quality of our products. We also maintain a larger manufacturing capacity for diverse dosage forms and the technology and expertise to produce custom formulations.

Independent & Well-equipped Quality Control Laboratory capable to carry out analysis of raw materials, in-process samples, finished products, stability samples, packaging materials, microbiological analysis, etc. and ensures documentation of the same.

Maxtar has a qualified affairs team that guarantees that the company concedes with all regulations and laws in force time to time. They provide strategic guidelines right from the commencement of the development of a product, thus making an essential commercial contribution in helping the company to produce high-quality , righteous products. Our Regulatory Team is capable of all types of regulatory submissions (ACTD, CTD, eCTD) Holistic support through various regulatory activities like DCGI/FDC filing Pharmacovigilance, import/Export, Licensing, legal, etc.
Maxtar has team of 10 personnel which is continuously working on dossier preparation as per regulatory requirements whether it is CTD or eCTD format
We have collaborated with Pex Print for the packaging of pharmaceuticals. Pex Print provides the Best quality packaging services for pharma products. Best packaging like ALU-ALU and Blister packaging is offered at In house packaging facilities. Pex Print also offers Cartons, boxes, cases, bags, and other packing materials.
